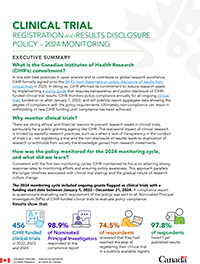
Clinical Trial Registration and Results Disclosure Policy
2024 Monitoring – Executive Summary
CIHR's Monitoring of Clinical Trials Registration and Disclosure – 2024
The Canadian Institutes of Health Research (CIHR) has committed to clinical trials transparency
In line with best practices in open science and to contribute to global research excellence, CIHR formally signed onto the WHO Joint Statement on public disclosure of results from clinical trials in 2020. In doing so, CIHR affirmed its commitment to reduce research waste by implementing a policy guide that requires transparency and public disclosure of CIHR-funded clinical trial results. CIHR monitors policy compliance annually for all ongoing clinical trials funded on or after January 1, 2022, and will publicly report aggregate data showing the degree of compliance with the policy requirements. Ultimately, non-compliance can result in withholding of new CIHR funding until compliance has been achieved.
Why monitor clinical trials?
There are strong ethical and financial reasons to prevent research waste in clinical trials, particularly for a public granting agency like CIHR. The real-world impact of clinical research is limited by wasteful research practices, such as when a lack of transparency in the conduct of trials (i.e., not registering a trial and the non-disclosure of results) leads to duplication of research or withholds from society the knowledge gained from research investments.
How was the policy monitored for the 2024 monitoring cycle, and what did we learn?
Consistent with the first two monitoring cycles, CIHR maintained its focus on attaining strong response rates to monitoring efforts and ensuring policy awareness. This approach parallels the longer timelines associated with clinical trial start-up and the gradual nature of research culture change.
The 2024 monitoring cycle included ongoing grants flagged as clinical trials with a funding start date between January 1, 2022 - December 31, 2024. A compliance report (a questionnaire evaluating each requirement of the policy) was sent to all Nominated Principal Investigators (NPIs) of CIHR-funded clinical trials to evaluate policy compliance. Results show that:
Long description
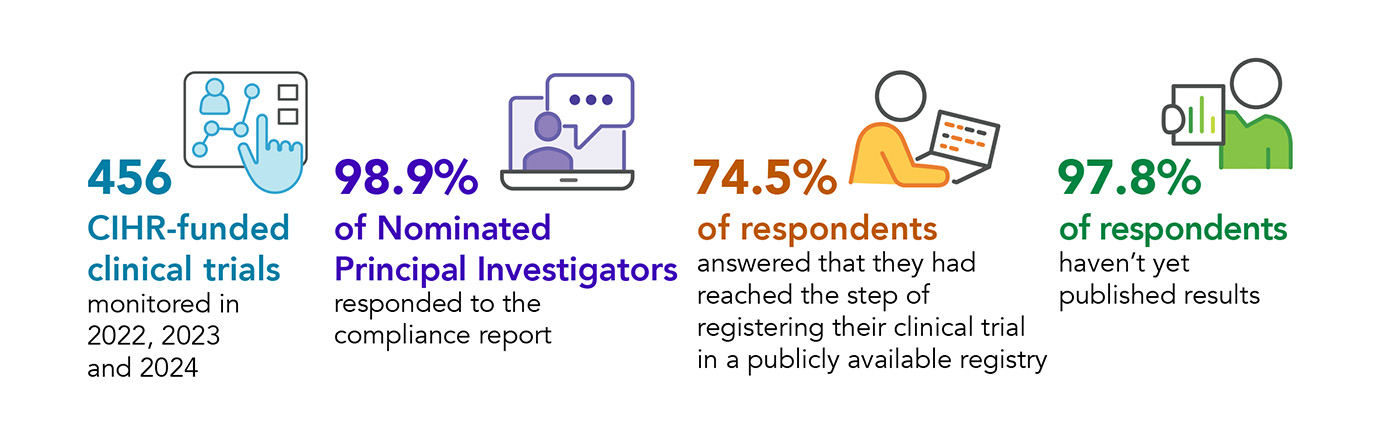
- 456 CIHR-funded clinical trials monitored in 2022, 2023 and 2024
- 98.9% of Nominated Principal Investigators responded to the compliance report
- 74.5% of respondents answered that they had reached the step of registering their clinical trial in a publicly available registry
- 97.8% of respondents haven't yet published results
- 456 trials, which were funded by CIHR between 2022 and 2024, were monitored this year.
- Compliance reports were received for 451 of the 456 clinical trials, a 98.9% response rate, over the course of the monitoring cycle (including follow-up and escalation).
- 326 (70.1%) NPIs confirmed that they had registered their clinical trial in a publicly available, free to access, searchable clinical trial registry complying with WHO's international agreed standards before the first visit of the first participant. Another 10 respondents answered "yes" to the question of trial registration, but did not use a WHO-approved registry, or did not specify the registry used. The remaining respondents indicated they were in the early stages of trial start-up and working towards registration.
- Ten (10) NPIs reported publications. Of these, 6 NPIs reported more than one publication for a total of 26 reported publications. These included publications of trial results, some from studies that were ongoing prior to monitoring, a protocol publication, and other non-trial result publications. All other respondents had not yet reached the publication stage.
Although 25.5% (115) of respondents had not reached the registration step by the final reporting deadline, many of these studies had achieved milestones which precede trial registration, such as having applied for research ethics board and regulatory approval or awaiting approval from the registry, all rate-limiting steps associated with study start-up. Furthermore, registration rates were higher in trials funded in 2022 (88.6%) than in 2023 (76.8%), and those funded in 2024 (56.3%), indicating that compliance with this reporting element improves over time.
Few studies were sufficiently underway to report on results. Of the 10 studies that did report publications, 5 reported findings from trial activities conducted prior to the monitoring period, suggesting that these were trials already underway prior to receiving the CIHR grant being monitored. Additionally, some studies reported protocols, conference abstracts or publications that were pending, and not all the reported publications were open access. The broad range of responses for this reporting element, including responses that were not aligned with policy requirements, indicates that further engagement is required to support researchers in becoming fully compliant.
In this third year of monitoring, success in executing a coordinated, efficient, and thorough data collection effort can largely be attributed to the implementation of lessons learned from the first two years of monitoring. Despite the important increase in the number of trials monitored, a strong response rate was sustained.
We would like to thank the researchers and their institutions for their timely responses.
What's next?
As part of its ongoing efforts to promote a research culture of open science, CIHR continues to engage with the small number of NPIs who did not submit the compliance report by the reporting deadline to identify barriers and to work with them towards compliance. Additional education for NPIs and edits to compliance report questions are planned to promote accurate and complete reporting.
In line with the aim to work collaboratively towards compliance and to deliver an efficient monitoring process, CIHR gathered feedback from NPIs about their reporting experience this year, to support process improvement. General information about the policy continues to be communicated with partners, the research community and the public. CIHR will continue to monitor compliance and report on these results annually.
For additional information, please contact clinicaltrials-essaiscliniques@cihr-irsc.gc.ca.
- Date modified: