Better drug delivery, improved cancer therapies—and a path to a new generation of vaccines

Groundbreaking treatments like targeted cancer therapies and mRNA-based vaccines would not have been possible without almost five decades of fundamental research led by Dr. Pieter Cullis, a biochemist at the University of British Columbia. It all started with scientific curiosity and tiny bubbles of fat.
In the late 1970s, Dr. Cullis began studying the basic components of the fats that make up the membranes around our cells with funding from what is now the Canadian Institutes of Health Research. He used these components to create lipid nanoparticles, tiny biological envelopes that could deliver medication into our bodies.
At the time, 99.9% of chemotherapy drugs caused toxic side effects and only a small amount would reach cancer cells. Clinical trials showed that cancer drugs delivered through lipid nanoparticles targeted the tumour more directly and caused fewer side effects.
These results led the U.S. Food and Drug Administration (FDA) and the European Medical Agency to the approve Myocet and Marqibo, which are used to treat breast cancer and lymphoblastic leukemia. Today, many cancer drugs still use this method.
In the 2000s, researchers and pharmaceutical companies were developing new drugs based on ribonucleic acid (RNA). Dr. Cullis and collaborators showed that lipid nanoparticles turned out to be an ideal protective bubble to deliver RNA molecules into the cells. The treatment worked well for a rare liver condition called transthyretin amyloidosis and received approval in Canada, the United States, and Europe.
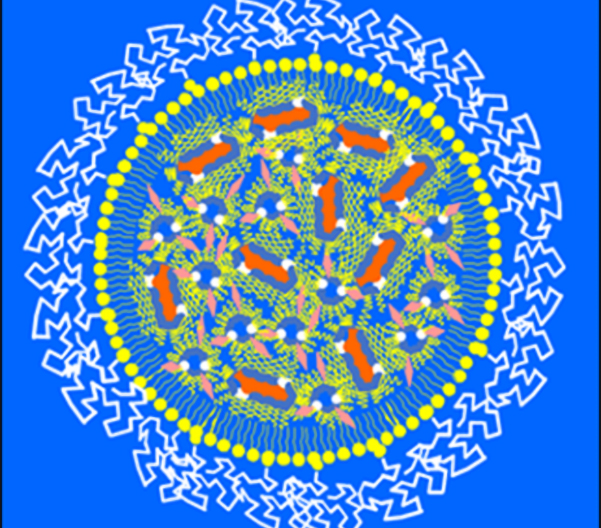
Then Dr. Cullis’s team tested if lipid nanoparticles could carry messenger RNA (mRNA), larger molecules with the ability to train cells to make missing proteins. “If a child is born not making a particular protein because of a genetic mutation, maybe our drug delivery method could get the liver to make the missing protein,” Dr. Cullis explained.
This research led to collaborations between Dr. Cullis’s UBC lab and a company he co-founded called Acuitas with the University of Pennsylvania and subsequently BioNTech (Germany) to develop mRNA-based vaccines for Zika and the flu. These teams used lipid nanoparticles to deliver mRNA coding for a virus surface protein to trigger an immune response.
When the COVID-19 pandemic hit, Pfizer-BioNTech and Moderna employed the same technology to rapidly develop effective mRNA vaccines that saved millions of lives. The rest became history.
What is so special about lipid nanoparticles is their limitless potential to enable emerging RNA-based therapies to treat and prevent diseases, making this a revolution in health research:
“The applications of RNA medicines are just enormous in disease treatment and prevention. Essentially any human disease can potentially be treated using these medicines. It’s a great time to be working because researchers can go after a disease in a very direct way. With these available tools, you can really have an impact,” says Dr. Cullis.

With continued CIHR funding, Dr. Cullis’s team is working to improve lipid nanoparticles to increase the impact of the groundbreaking medication they support. The team is also studying a way to trigger a release of cancer drugs directly in the affected area to further reduce severe side effects.
Dr. Cullis explains that these past and recent achievements would not have been possible without an incredible team and an entrepreneurial mindset. What initially started with assembling lab equipment in a coworker’s garage and offering free beer to staff every Friday later developed into 12 biotech companies, over 100 patents, and several university-business collaborations.
Dr. Cullis was appointed to the Order of Canada in 2024 for his contributions to the advancement of biomedical research and drug development, and for his mentorship of the next generation of scientists and entrepreneurs.
At a glance
Issue
In the early 1980s, only a small part of chemotherapy drugs could reach cancer cells, with the rest causing toxic side effects. There was a need for a more effective way to deliver drugs directly into cells.
Research
A solution inspired by nature to deliver life-saving therapies revolutionized drug development, changed the course of medical history, and saved millions of lives. By packaging medication inside tiny bubbles of fat, Dr. Cullis’s team helped researchers around the globe develop safer and more effective cancer therapies, vaccines and gene therapies. His technology was essential to delivering mRNA-based therapies such as the COVID-19 vaccines into our cells.
- Date modified: